App World
My stream

Chemical Equilibrium
Chemical Equilibrium is a tool for accurate estimation of concentrations and pressures of the chemical reactio...
$ 1.990 $ 2.990

Store review
Chemical Equilibrium is a tool for accurate estimation of concentrations and pressures of the chemical reaction reactants and products at equilibrium. The application derives final, equilibrium parameters based on equilibrium constants (Kc and Kp), initial concentrations and stoichiometric coefficients. Additionally, app calculates reaction quotient for any given concentrations and stoichiometry.
For a general chemical reaction:
aA + bB = cC + dD,
the Reaction Quotient (Q) is defined by:
Q= (C^c * D^d) / (A^a * B^b)
where A, B, C, D – are compounds’ concentrations or pressures and a, b, c, d are stoichiometric coefficient*. Sum of powers determines the reaction order.
*Importantly, real system powers may differ from reaction coefficients, due to overall reaction mechanism complexity.
If Q is not equal to reaction equilibrium constant K (Kc for concentrations and Kp for pressures), then reaction is not at equilibrium and it will proceed (due to difference in forward and reverse reaction speeds) to the direction defined by Q.
If Q is less than K reaction will move towards products (C and D), in opposite case – reverse reaction will prevail.
Example:
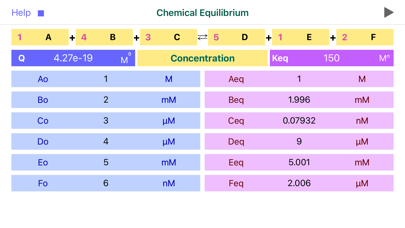
For given reaction: 2A + 1B = 3D + 2E , initial reaction concentrations are Ao=0.1M, Bo=0.2M, Do=0.3M and Eo=0.4M and Kc=1 M^2.
the Reaction Quotient (Q) is defined by:
Q= (D^3 * E^2) / (A^2 * B)
Loading the data to “Chemical Equilibrium” app, Reaction Quotient is immediately calculated, giving Q= 2.16 M^2. Apparently Q>K, suggesting that reaction is too close to the products side and reverse reaction will prevail to bring the reaction back to the equilibrium.
The new equilibrium concentrations of reaction components can be derived from the equation:
Apparently, solving this equation is rather demanding task, that turns to be unnecessary, since “Chemical Equilibrium” app immediately returns the answer: Aeq= 118.5 mM, Beq= 209.2 mM, Deq= 272.3 mM and Eeq= 381.5 mM.
The application features:
•To start calculation user is required to fill in stoichiometric coefficients, initial concentrations (pressures) of available components, and equilibrium constant Kc (Kp).
•Reaction coefficients are actually representing powers and therefore define reaction order.
•If certain compound do not contribute to equilibrium calculations, like water in aqueous solution, then to exclude the compound it is required to long press on its name field. To signal about the change, the background colour of the non contributing field is altering. To revert compound to the normal state it is required to long press again the appropriate field. See the screenshot.
•To start calculation initially or after updating the concentration or coefficient fields, user is requested to tap Run button!
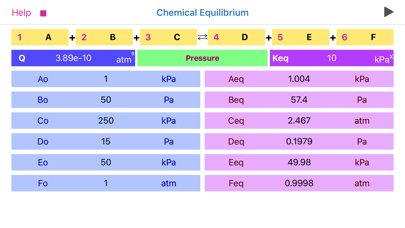
•Significant attention should be paid to concentration, pressure and equilibrium constant units. App automatically changes the set of available units as per order of reaction!
•The power of equilibrium constant units depicted as “x” is defined as (c+d-a-b), where a, b, c and d are the stoichiometric coefficients of the reaction.
•The basic conversions are as follows:
1 M= (1e3) mM
1 atm=101325 Pa
Size
8.0 MB
Last update
Nov. 28, 2019






 Facebook
Facebook Twitter
Twitter Google plus
Google plus